acids & bases
Proton Transfer
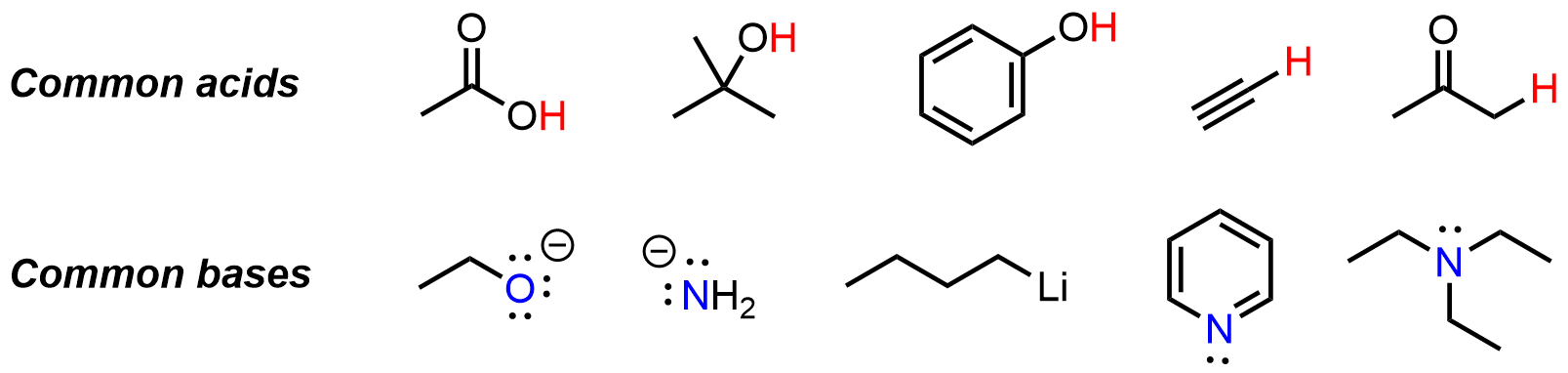
Organic acid-base chemistry focuses on the behavior of molecules that can donate or accept protons (H⁺) in organic systems. Like in General Chemistry, acids are species that donate protons, while bases accept them, according to the Brønsted–Lowry definition. Common organic acids include carboxylic acids, phenols, and alcohols, while amines and alkoxides are often used as bases. The strength of an acid or base is quantified by its pKa value: a lower pKa (< 0) indicates a strong acid, while a higher pKa corresponds to a weaker acid.
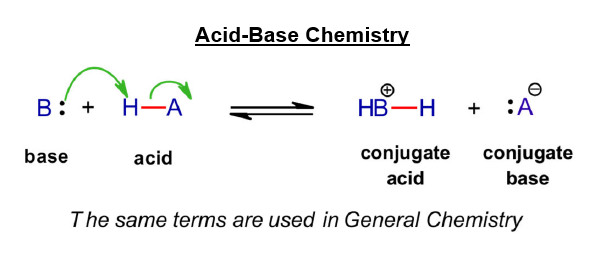
Acid-base reactions in organic chemistry are influenced by several factors: electronegativity of the atom attached to H, which affects how electron-poor the proton is; resonance stabilization in the conjugate base, which stabilizes the negative charge through delocalization; and inductive effects, where electron-withdrawing groups enhance acidity by pulling electron density from the proton while also stabilizing the conjugate base. Solvent polarity also plays a critical role, with polar solvents helping to stabilize charged species. The same terms are used from General Chemistry where the base and acid react to give the related conjugate acid and conjugate base (below).
The mechanism arrows used in acid-base chemistry are simple; one arrow to show a bond forming between base and proton, in which the lone pair from the base becomes a bond pair, and a second arrow to show the bond between proton and conjugate base breaking. This describes a simple bimolecular concerted process, which is often the first mechanism studied in the Organic courses.
Bronsted-Lowry acids have an electron-poor proton, which is usually attached to an electronegative element. In organic chemistry we greatly expand the acid collection beyond what you have seen before in General Chemistry. Bronsted-Lowry bases have an electron-rich atom, which has at least one lone pair associated with it. The lone pair is able to be donated to the electron-poor proton so that the system may become more stable overall.
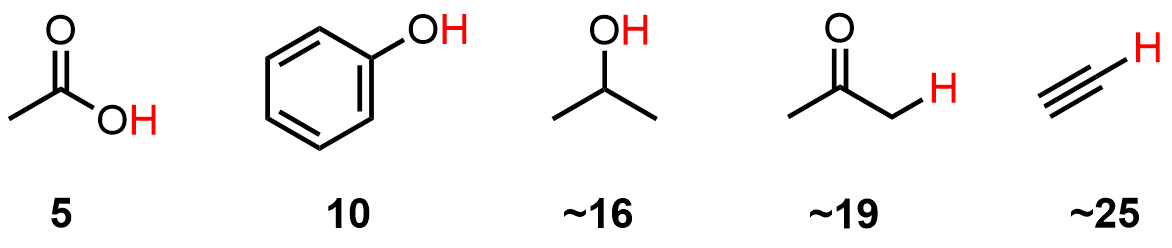
Any acid will have an acid constant, the Ka, which is a measure of its ability to give up a proton. These numbers are difficult to work with so we convert them to pKa (a log derivative). You will find that knowing these numbers is very helpful when solving problems later. The basic ideas behind pKa values, and examples of applications, are given below.