Preparing for Organic 1 - the transition from general chemistry
A summary of General Chemistry basics (PDF)
Click on each section heading to read/listen to the contents. Images within are clickable, either to expand or link to useful resources.
More detail on prep
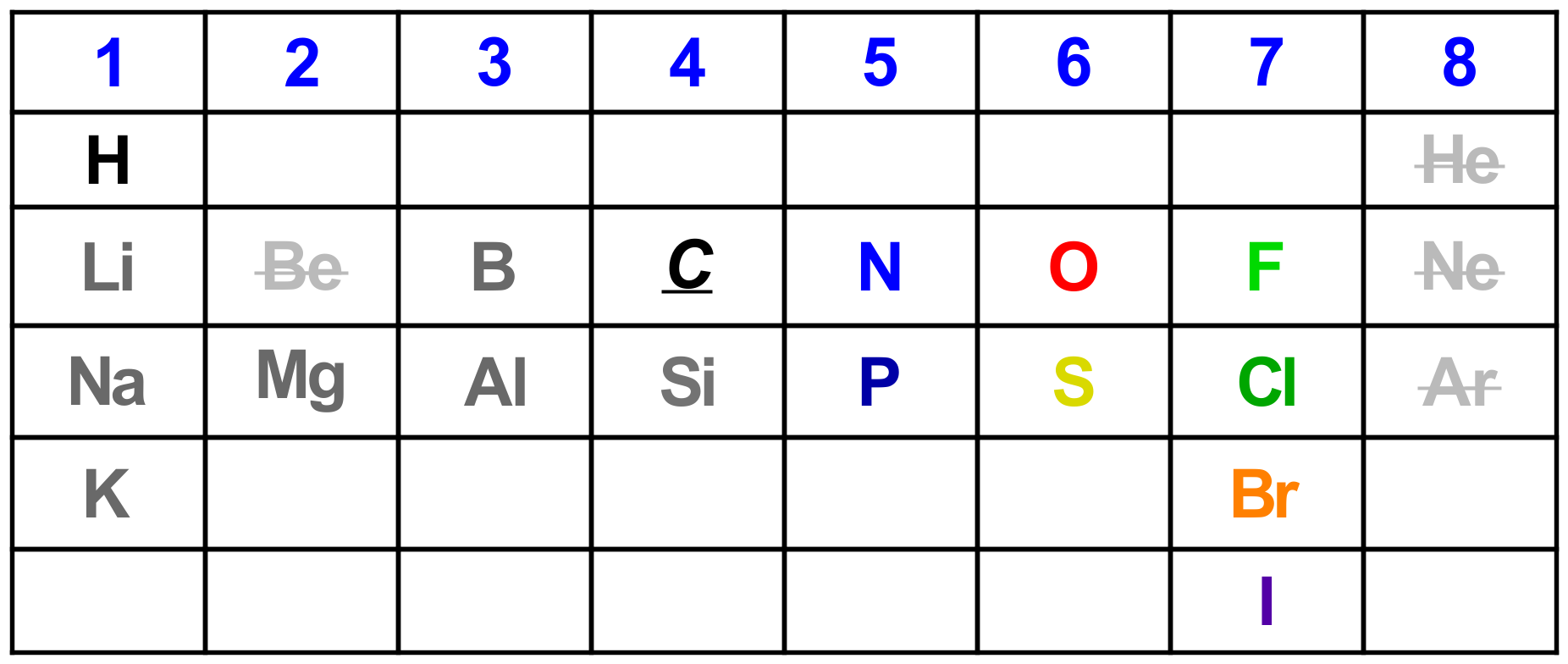
Periodic Table: You need to have a good understanding of why the periodic table is organized the way it is. Knowing roughly where the main atoms reside in the table will be a huge help.
You don’t need to know all of the elements; to get started you should focus on the top of the table and elements that actually get used early on in Organic 1. Elements (e.g. transition metals) will be added later as they are needed for new reactions.
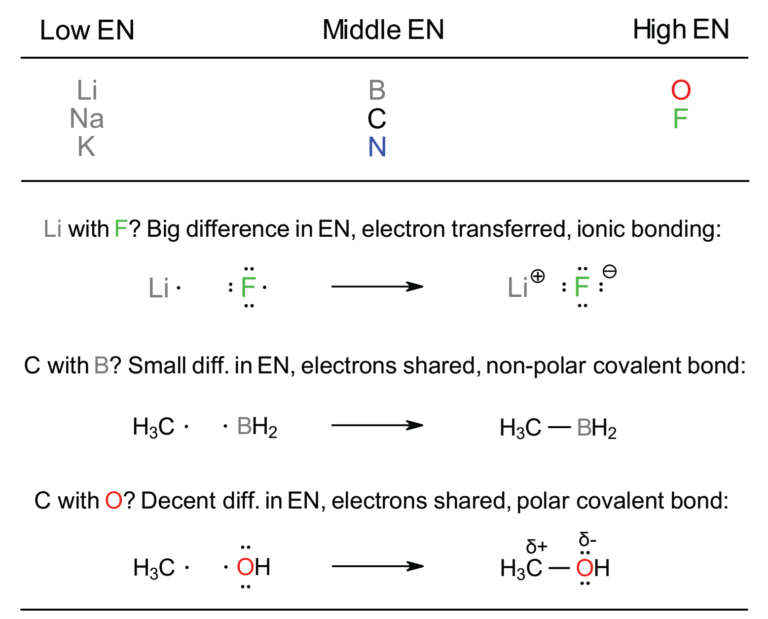
Focus on Carbon being in the middle of this abbreviated table; this is important as C has a “middle” electronegativity (2.5). Elements to the left of C are less electronegative; those to the right are more electronegative. This will dictate types of bonds formed.
Metals on the left give away electron(s) to form ionic bonds, those in the middle share, those on the right can accept electrons or share to form ionic or covalent bonds. You learned this in General Chemistry and it still applies in Organic Chemistry.
Before you begin Organic 1 you should know where these elements are on the table and why they are organized this way. This will save time when making decisions on how the elements will interact based on their electronegativities and whether they can share of transfer electrons. If you are looking at a periodic table during an exam to find out where an element is, you are wasting time that should be spent elsewhere.
Notice that some elements are crossed out. We don’t talk about Berylium (it’s rare) and we don’t make molecules from the inert (Noble) gasses (because they are inert with a full octet). The rest of the elements in the above table will do what they did in General Chemistry so you should be on top of that as you prepare for Organic 1.
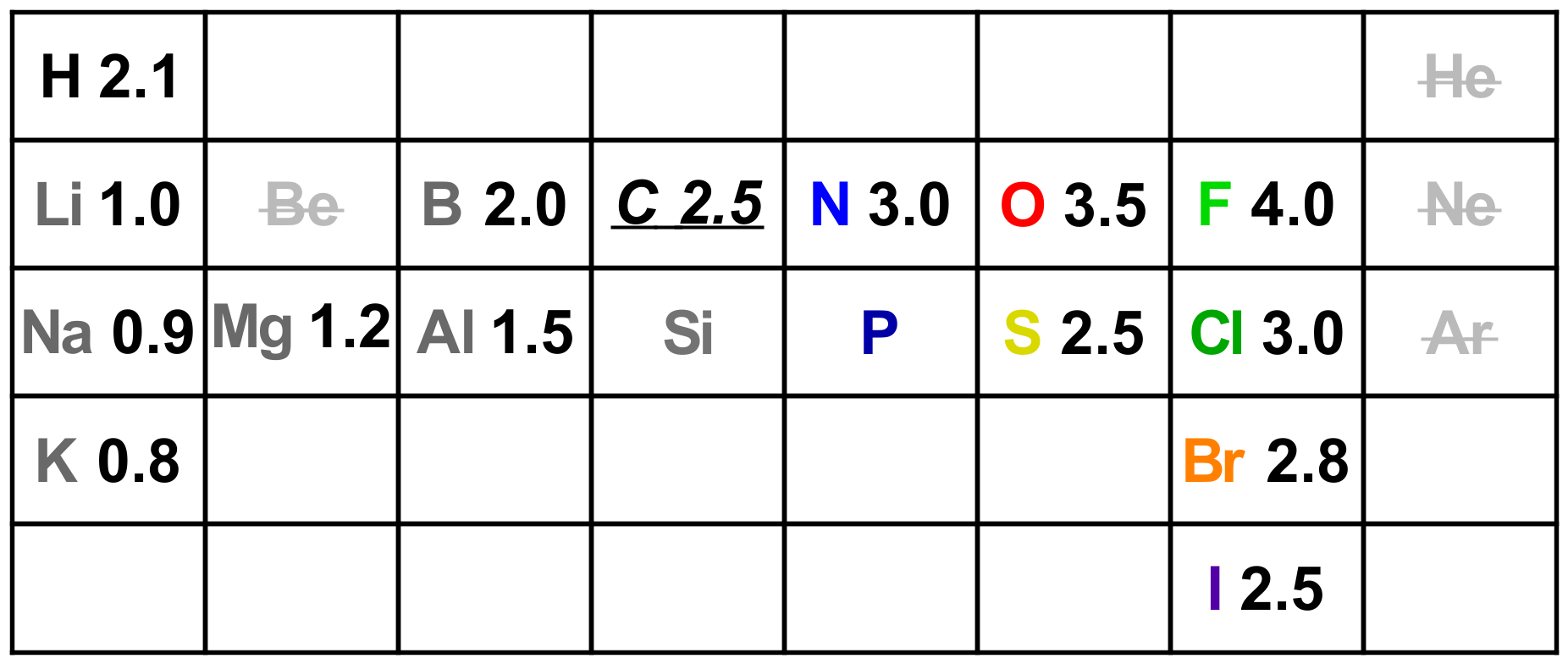
Electronegativity: This idea, which you first studied in General Chemistry, is so important that it will help to know some actual numbers. Instead of just learning a general trend, by being more specific you will be able to solve problems more confidently.
Electronegativity (EN) of elements increases from left to right in the Periodic Table in ~0.5 increments heading to F, which has the highest electronegativity value of 4.0 (on the 4.0 scale). Going from left to right, valence electrons are added to the same energy level while the nucleus gains one extra proton per group. The more positive nuclei will attract the valence electrons more tightly, which affects atomic size and then reactivity. Going down the Table the valence electrons get further away as electrons go into higher orbitals (3s, 4s, etc.) so the influence of the nucleus fades.
Pauling Scale electronegativity values for the important early elements.
Knowing the numbers means you are able to decide what kind of bonding will occur in between different atoms. Very different EN values, e.g. Na @ 0.9 and Cl @ 3.0 are only ever forming ionic bonds. C @ 2.5 and H @ 2.1 will only form covalent bonds with each other through sharing of valence electrons. There is no absolute cutoff difference, however.
Once molecules are formed, you will then be able to decide which areas within are non-polar or polar, which is essential for understanding reactivity patterns. Anions will be electron-rich and go after electron-poor atoms in polar-covalent areas.
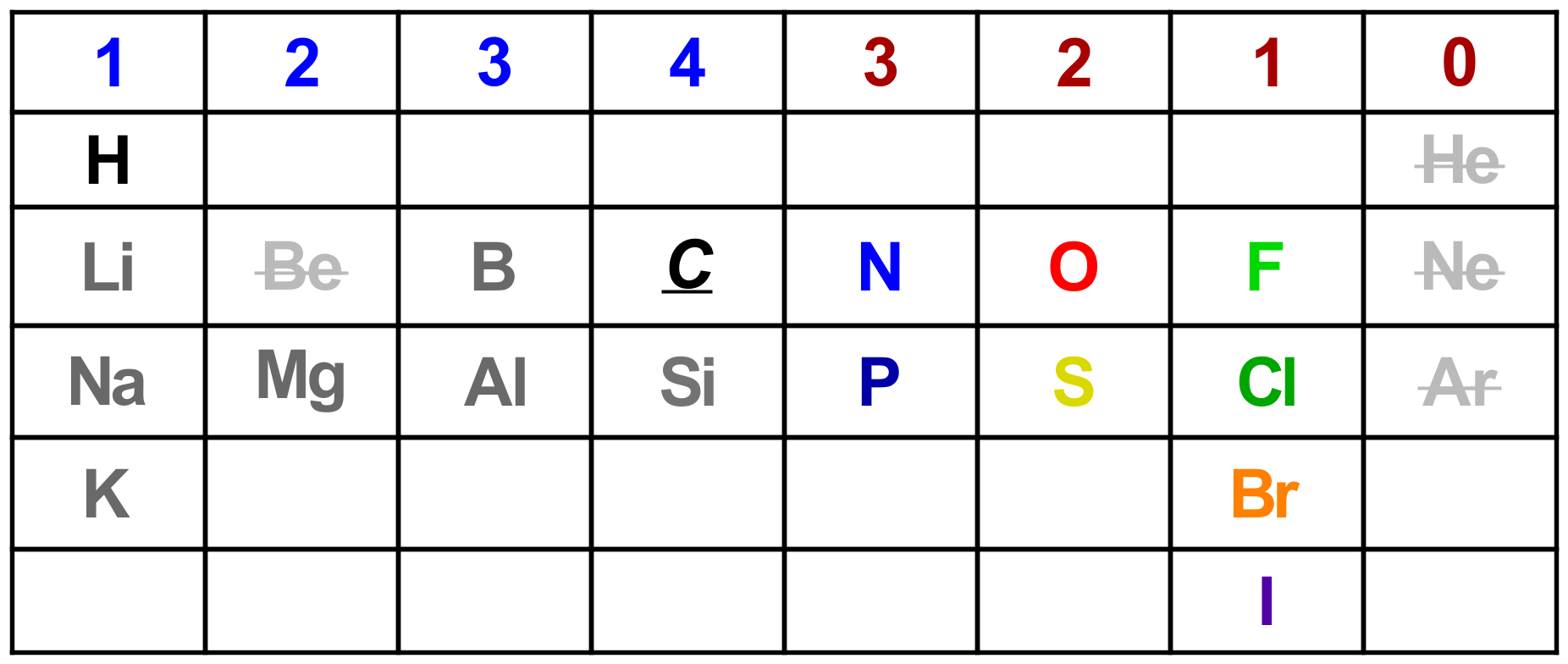
Valence: This comes straight from the Periodic Table and having a working knowledge of it is essential. An atom with one electron in its valence shell will form one bond, most likely by giving that electron away (think Li, Na, K). Atoms with seven electrons will form one bond to gain the octet (think F, Cl, Br). An atom with four valence electrons (carbon!) will need to form 4 bonds maximum to achieve the octet.
Electronegativity (EN) of elements dictates what type(s) of bonds are formed but the valence atomic structure governs how many. The table below gives an idea of what to expect. Just remember that 8 is the desired number for most of the upper key elements. Smaller atoms (H, Li. etc.) don’t need 8 as 1s shell can be filled to give a full valence.
Number of bonds formed by each of the important early elements.
Carbon is very flexible, due to its middling EN. It forms 4 bonds in its stable (neutral) molecules but will be able to cope with three bonds temporarily in intermediates. C will never form 5 bonds in Organic 1 or 2. Boron (B) and Aluminium (Al) are unique at this stage in that they form 3 bonds in neutral molecules but will form 4 (for the octet) during the various reactions in which they feature. They are used as Lewis Acids.
Atoms to the right of C will have lone pair(s) in their neutral molecules and will be able to share with other atoms to form common species such as hydronium or ammonium in which the central O or N atom has a positive charge. Same as in General Chemistry.
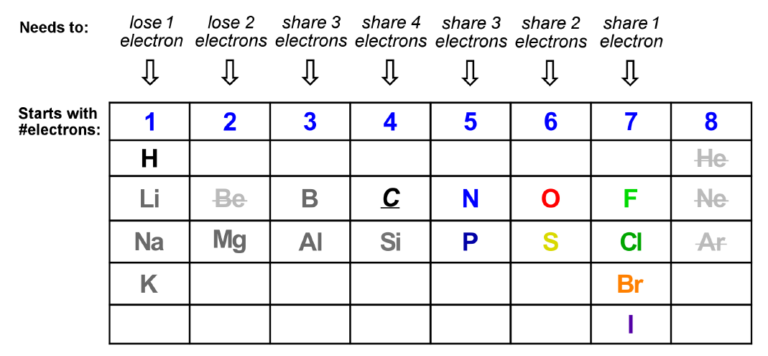
Octet Rule: Almost all of the elements used early in the undergraduate Organic Chemistry sequence are at the top of the periodic table and thus are in search of the perfect electronic “octet” in their valence shell.
The Periodic Table is an organization of elements in their atomic form before they react to form molecules. Apart from the Nobel (inert) gasses, which already have the complete valence shell, elements lose or share electrons to match the Noble gasses.
On the left of the table (think Li, Na, Mg), atoms have 1 or 2 valence electrons (and low EN values) so it will be easier to lose electrons than share or pick them up. Atoms on the right (think O, F, Br) will pick up electrons to achieve the octet. Those in the middle will share electrons in covalent bonds. How many electrons each atom needs depends on how many they have to begin with, which comes from the Periodic Table.
Don’t forget about non-bonding electrons (lone pairs, l.p.), which count towards the octet: N (also P) has 1; O (also S) has 2, F (also Cl, Br, I) has 3.
How each element will get to the nearest Noble gas octet.
How an atom gets to the octet is a function of its own electronegativity and what element(s) it is bonding to. Each of the elements above is capable of forming either covalent or ionic bonds depending upon the other elements involved.
Do not worry about elements that can expand their octet (S, P, etc.) at this point; that will come later in the Organic courses. For now the abbreviated table above shows you how each of the important elements will achieve the octet.
Bonding: Bonds are a result of the relative electronegativities of the atoms involved; very different electronegativities (e.g. Na and Cl) means ionic bonds through electron transfer, while close electronegativities means sharing in covalent bonds.
In General Chemistry you spent a lot of time studying ionic salts, their composition, and their stoichiometry in chemical reactions. While that material is useful it can also be confusing. In the Organic classes we start simple but we need to expand on your understanding of which bonds are most likely to be formed in various situations. Most of this relies on understanding relative electronegativity (EN) values (see earlier).
In the Organic courses we expand upon the basic idea of “ionic” and “covalent” bonds and invoke the “polar covalent bond” as being essential for reactivity. Ionic bonds will be obvious as they are formed between atoms on the left and right of the Table. When atoms are closer together (and thus have similar EN values) they share bond electrons in covalent arrangements. Some difference in EN values then leads to polar covalent.
The main bonding patterns seen in Organic Chemistry.
The question will be, “where are the electrons” in each bond? With large differences in EN the electron(s) will be associated completely with the more EN atom. For moderate differences the electrons are shared but closer to the more EN atom to give a dipole. For small EN differences the electrons are equally shared and no dipole exists.
More detail on preparing for the Organic sequence is found here.