Bond-Line Structures
Simplifying Representations
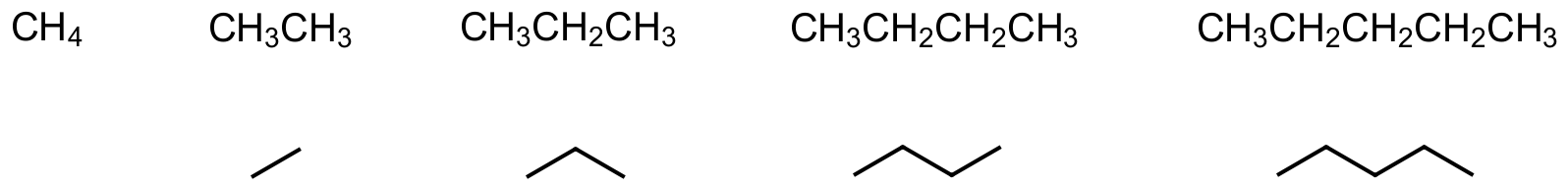
Bond-line structures, also called skeletal structures, are simplified representations of organic molecules commonly used in chemistry. In these diagrams, carbon atoms are implied at the ends and intersections of lines, while hydrogen atoms bonded to carbon are omitted for clarity. Each line represents a covalent bond, and multiple lines indicate double or triple bonds. Heteroatoms (such as oxygen, nitrogen, or halogens) and their attached hydrogens are shown explicitly. This notation provides a clear and efficient way to depict complex molecules, emphasizing connectivity and functional groups without clutter. Bond-line structures are widely used in organic chemistry for quick visualization; the C-1 through C-5 alkanes are shown here:
Organic molecules get big pretty quickly so it benefits us to strip out information when possible. In bond-line structures this means not including hydrogen atoms but remembering they are there when we consider the molecule’s potential reactivity.
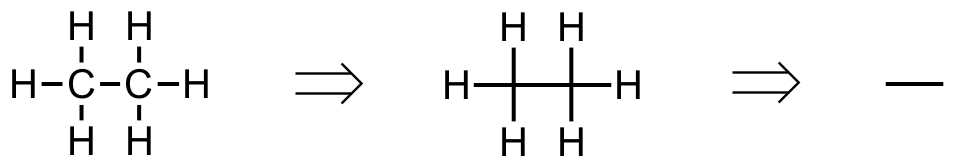
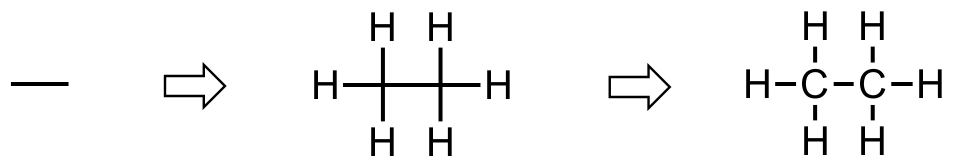
We learn how to strip out unneeded information early on so that most stuctures are drawn with just lines. Beginning with the alkanes, we find that ethane become just a line, however when reading that line we must recall two attached methyl groups.
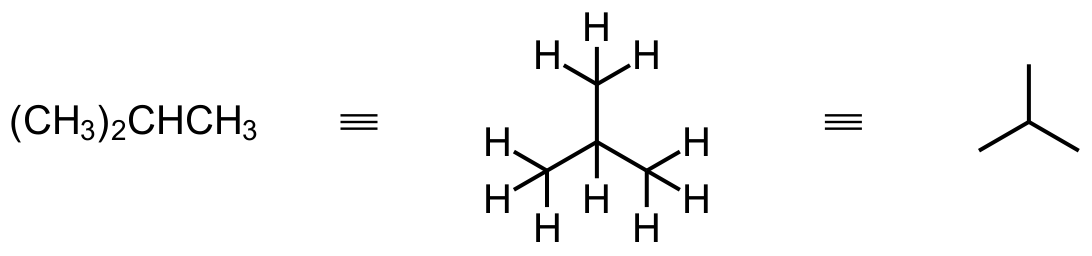
When branches are involved, we remember that carbon can only have 4 bonds at most and that any C atoms with 5 bonds will be incorrect. Here the connections between C atoms need to be worked out and reduced to single lines.
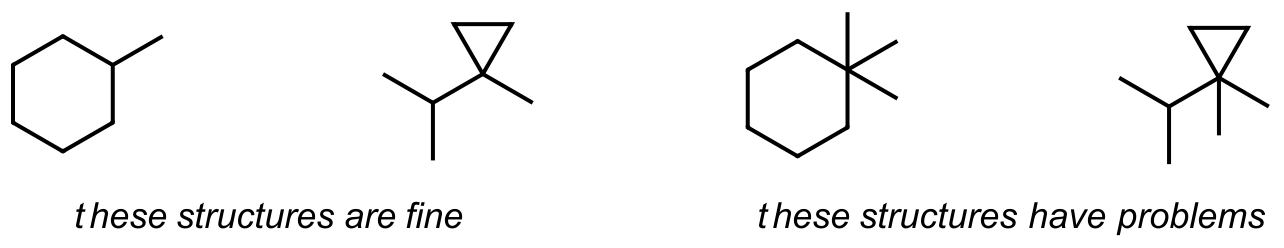
While this idea is fairly simple, it is easy to make mistakes by not paying attention to the number of bonds to carbon. Check those bonds once you have drawn a structure and avoid having too many bonds to anyone carbon atom.
Also, be careful when drawing structures so you don’t repeat. The following examples highlight this. If a drawing is the same when rotated through a plane, or it has the same name when you apply nomenclature rules, it is the same molecule.