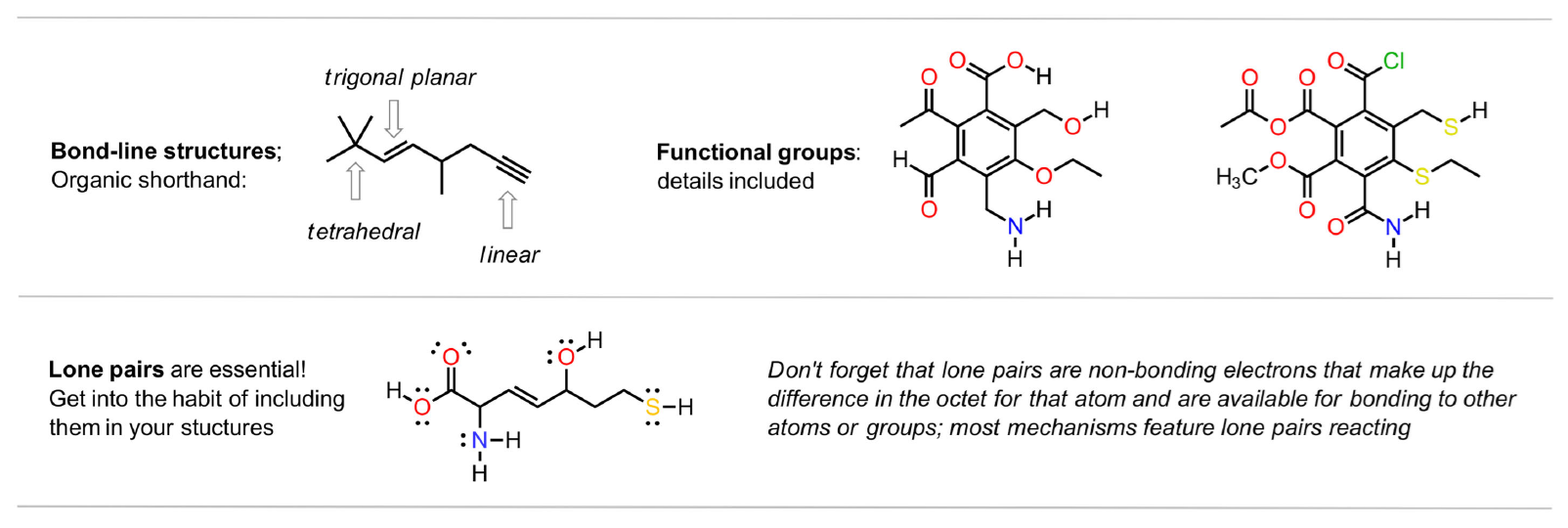
functional groups
Typical second semester groups
After establishing the simple functional groups in the first semester, alkenes, alkyl halides, alkynes, etc., the second semester typically focusses on groups that will be important later in Biochemistry and Polymer Chemistry. Aromatic compounds, aldehydes and ketones, carboxylic acids and their derivatives, as well as teh various types of amines feature heavily. Examples of typical second semester groups are organized below and both semesters are summarized in the video.
Aromatics
Aromatic compounds are a class of organic molecules that contain one or more benzene-like rings, which consist of atoms arranged in a planar, cyclic structure with alternating pi bonds. This arrangement creates a conjugated π-electron system that fits Hückel’s rule (having 4n + 2 π electrons, conjugated and planar), giving aromatic systems exceptional stability. They exhibit unique chemical reactivity, such as undergoing substitution reactions rather than addition, to preserve their aromatic stability. Common examples include benzene, toluene, and naphthalene. Such compounds find wide use as in organic synthesis; benzene is shown here.
Benzene
Aldehydes
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and an alkyl or aryl group. The general formula is R–CHO, where R represents an attached organic residue. Aldehydes are typically formed by oxidation of primary alcohols and are highly reactive due to the polar nature of the carbonyl group. They play an important role in organic synthesis, serving as intermediates in the production of alcohols, carboxylic acids, and other useful compounds. Aldehydes may exhibit distinctive odors, for example butyraldehyde, which has the smell of rancid butter. The structure is shown here.
Butyraldehyde
Ketones
A ketone is an organic compound that features the carbonyl group (C=O) bonded to two alkyl or aryl groups. The general formula for ketones is R–CO–R′, where R and R′ represent carbon residues. Unlike aldehydes, ketones do not have a hydrogen atom attached to the carbonyl carbon, which influences their reactivity. Ketones are commonly formed by the oxidation of secondary alcohols and are relatively stable compared to aldehydes. They play important roles as electrophiles in organic synthesis as well as biological processes, for example in metabolism. Ketones are widely used as solvents, as well as synthetic intermediates. The structure of cyclohexanone is shown.
Cyclohexanone
carboxylic acids
Carboxylic acids are organic materials that contain the carboxyl group (–COOH), which consists of a carbonyl (C=O) and a hydroxyl (–OH) group bonded to the same carbon atom. Their general formula is R–COOH, where R represents an alkyl or aryl residue. Carboxylic acids are generally formed by the oxidation of primary alcohols or aldehydes. They are weakly acidic, with pKa around 5, due to the electron-withdrawing carbonyl group, which also effectively stabilizes the anionic conjugate base. Carboxylic acids are widely used in food preservation, medicinal chemistry, polymers, and as intermediates in organic synthesis. Benzoic acid is shown here.
Benzoic acid
acid (ACYL) Halides
Acyl halides are reactive organic compounds derived from carboxylic acids by replacing the hydroxyl group (–OH) with a halogen atom (most often Cl or Br). Their generic formula is R–COX, where R is an alkyl or aryl group and X is a halogen. The carbonyl group (C=O) in acyl halides is polarized, making them excellent electrophiles in many nucleophilic acyl substitution reactions. They are commonly used as intermediates in organic synthesis for producing esters, amides, and anhydrides. Due to their reactivity, acyl halides are not found in Nature and must be handled carefully, as they can hydrolyze rapidly in the presence of water. Propionyl chloride is featured.
Propionyl chloride
Acid anhydrides
Related to acid halides, acid anhydrides are organic intermediates derived from carboxylic acids by removing water between two COOH groups, forming a structure with two acyl groups bonded to the same oxygen atom. Their general formula is (RCO)₂O, where R represents alkyl or aryl groups. Acid anhydrides are highly reactive due to the presence of two electron-withdrawing carbonyl groups, making them useful electrophiles in nucleophilic acyl substitution reactions. They are used in organic synthesis to give esters and amides. Anhydrides readily hydrolyze in the presence of water to regenerate carboxylic acids. Acetic anhydride is shown here.
Acetic anhydride
esters
Esters are formed by the reaction between a carboxylic acid and an alcohol, often with acid catalysis, and with the elimination of water in a condensation process. They contain the general structure –COOR. The ester motif includes a carbonyl (C=O) bonded to an oxygen atom that is then attached to a carbon chain or cycle. Esters are generally much less reactive than acyl halides or anhydrides, since they are less electrophilic and OR is a worse leaving group. They do undergo hydrolysis in acidic or basic conditions to regenerate the parent acid and alcohol. Esters are used in fragrances, flavorings, as solvents, and as synthetic intermediates. Ethyl acetate is pictured here.
Ethyl acetate
amides
Amides are derived from carboxylic acids by replacing the hydroxyl (–OH) with an amino group (–NH₂, –NHR, or –NR₂). General formula is R’–CONR₂, where R’ represents an alkyl or aryl group. The structure includes a carbonyl (C=O) bonded to N, which allows for significant resonance stabilization and reducing C=O reactivity compared to acyl halides or anhydrides. Amides are important in both synthetic and biological chemistry, forming the primary backbone of proteins as peptide bonds. They are highly stable linkages, making them suitable for connecting monomers in polymers and as useful motifs in drug and materials synthesis. The solvent DMF is shown.
N,N-Dimethylformamide
amines
Amines are compounds derived from ammonia (NH₃) by replacing hydrogen atoms with alkyl or aryl groups. They may be primary (–NH₂), secondary (–NHR), or tertiary (–NR₂) based on the number of substituents attached to nitrogen. Amines act as bases due to the lone pair of electrons on nitrogen, making them important as proton sponges. They also serve as good nucleophiles in processes such as nucleophilic acyl substitution and reductive amination. Amines play important roles in biological systems such as amino acids, neuro-transmitters, and various pharmaceuticals. Amines are used in dyes, polymers, and various materials. Triethylamine is pictured here.
Triethylamine