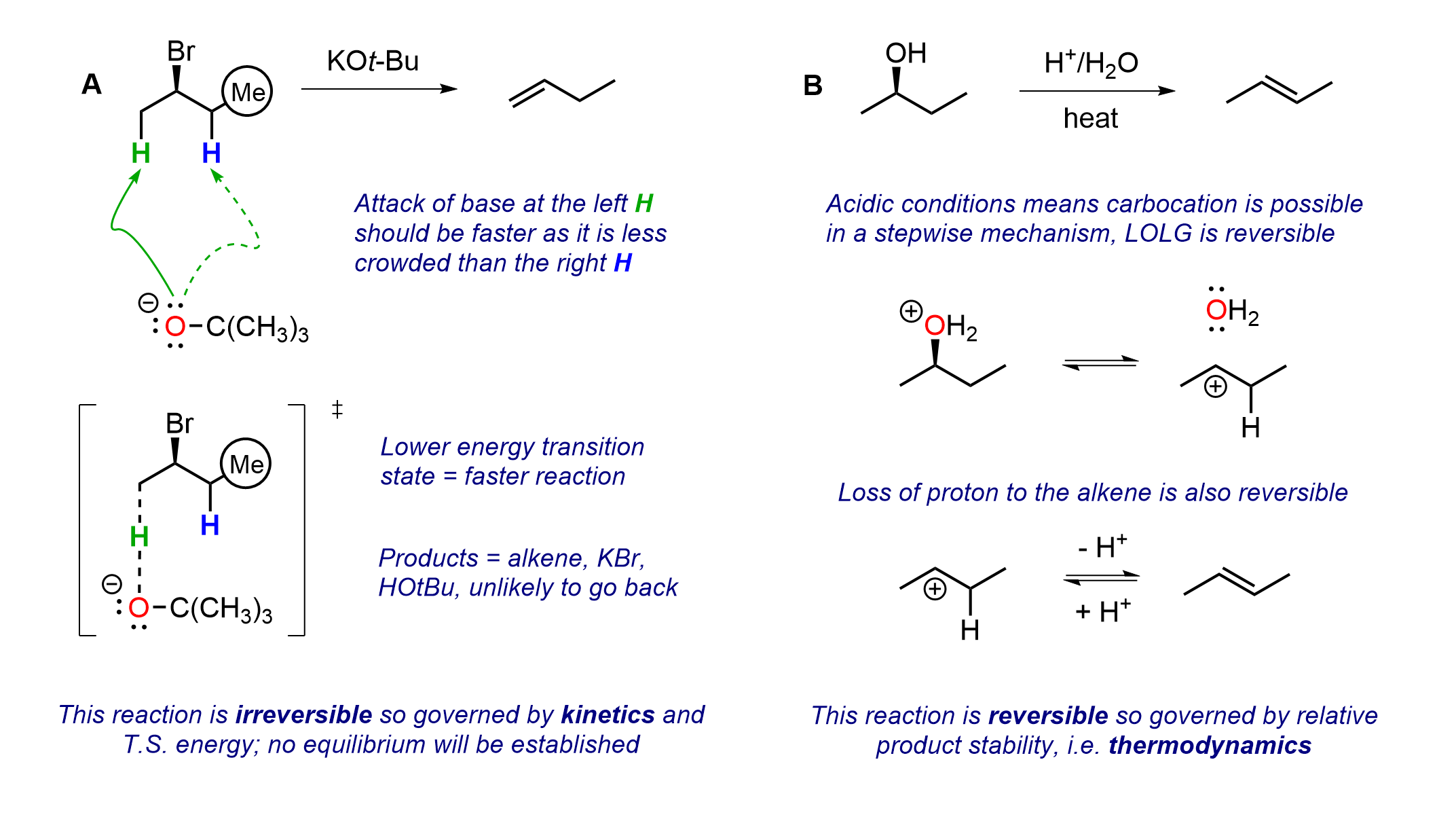
To get beyond rote memorization in a mechanism it is useful to be able to analyze each step in a reaction to decide if it is governed by kinetic or thermodynamic factors or, in some cases, both. Is the product distribution of a process governed by relative transition state energies, relative product stability, or both? The two reactions here highlight these ideas. In Equation A the environment is basic with a large base being employed; this results in the less substituted Hofmann alkene as the major product. In Equation B the environment is acidic, and the outcome favours the more substituted Zaitsev alkene being the major product.
Consider the following comparison of these elimination reactions in an exam question format.
Reaction A uses an alkyl halide and a large base so we expect Hofmann regioselectivity. The base is trying to transfer the negative charge from the less stable O to the more stable Br, however the alpha carbon is blocked and SN2 is shut down. The base diverts to go after a beta proton, with there being two options here. The left hydrogen is more accessible since the methyl group on the right will block the other H. This leads to a faster deprotonation via the lower energy transition state. This reaction is irreversible, mainly since the negative charge has been transferred from O to Br, so transition state energies and kinetics govern the outcome.
In Reaction B an alcohol is reacted with acid at high temperature and the more stable Zaitsev alkene is formed. Here the alcohol needs to be protonated first to make a better leaving group, a step that will be reversible. The leaving group is then able to break off, in another reversible step, in which the water can simply trap the carbocation to go backwards.
If the water behaves as a base instead of a nucleophile, it may take a beta proton and generate an alkene. That step will also be reversible since alkenes are known to add protons to give carbocations. If a proton is lost from the left the Hofmann alkene would form, however that could also pick up a proton and go backwards. Picking off the proton on the right gives the more stable Zaitsev alkene. Since all steps are reversible, material will find its way mostly to the more highly substituted alkene in a process governed by thermodynamics.