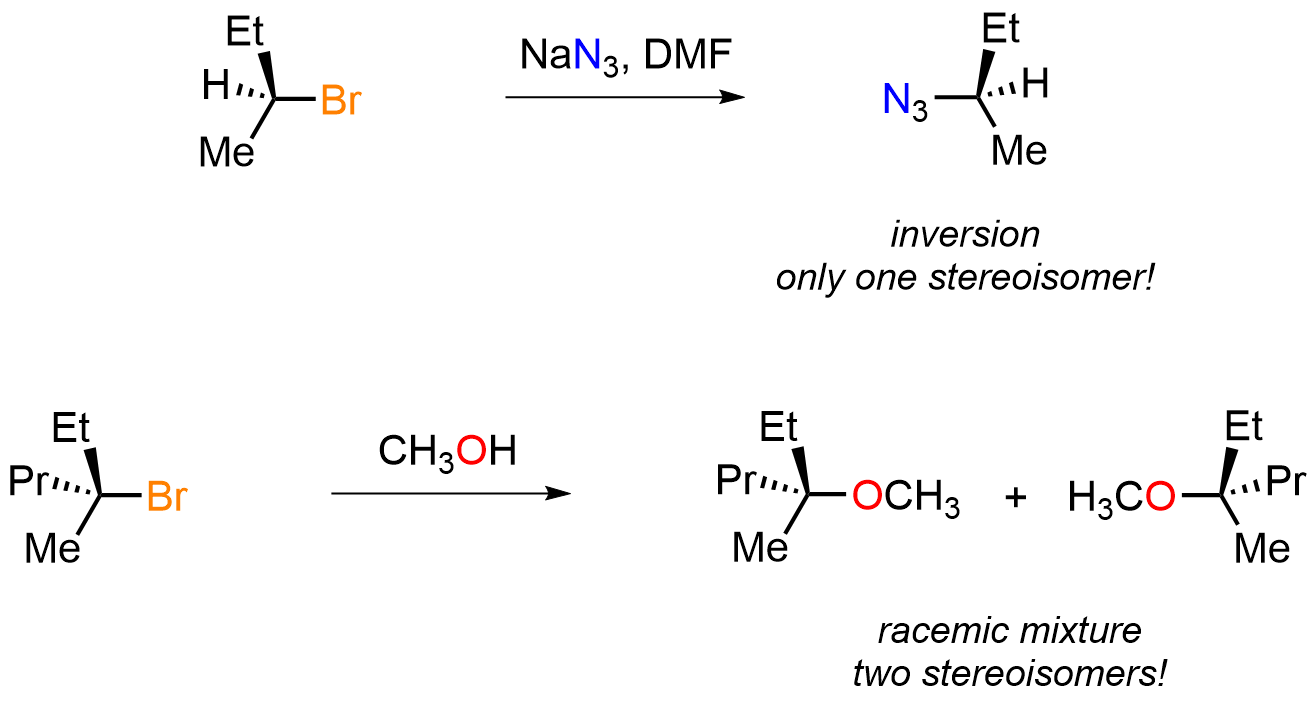
Stereochemical changes as a consequence of an organic molecule reacting are one of the most important clues as to how that reaction happened. Whether the result shows all possible stereochemical outcomes or a limited few is really helpful in beginning to map out how the changes occurred and whether the pathway was concerted or stepwise. For example, in the substitution reactions encountered early on, what does “inversion” versus “racemic mixture” formation tell us about the processes involved? Consider the two reactions below and what their differing stereochemical outcomes infer about how the products were formed.
In the first reaction we only get one product from a stereochemically pure secondary alkyl bromide and that product’s stereochemistry is the opposite of the starting material. Only one stereochemical outcome means the reaction is specific for one stereochemistry and not selective, which is where the term stereospecific comes from. The second reaction gives two stereoisomers in equal amounts from a stereochemically pure tertiary starting alkyl halide so that reaction is neither specific or selective.
The first reaction is somehow defined by the direction from which the incoming species (the nucleophile) approaches the electron-poor carbon (the electrophile) while the second reaction is not. Firstly, this tells us that the products cannot be formed in the same way in each reaction. To work out mechanisms we need to consider when bonds are formed and broken and what consequence that event (or events) will have on the molecule’s shape. Here the C to Br bond must break and the nucleophile to C bond must form in both cases.
In the first reaction the incoming group (nucleophile) is only attacking from one side while in the second reaction it is attacking from both sides. This means the bromine must still be attached as the nucleophile bonds in the first reaction but the bromine must be gone in the second process. That suggests a 3-valent, trigonal planar species is formed in the second reaction, which we know to be a carbocation that may then be attacked equally from either side to give a racemic mixture. In the first reaction the nucleophile must be kicking out the leaving group and forcing the attached alkyl groups to move in space, which we know to be the inversion process.