Shape & structure
Basic Molecular Geometry
Basic Shapes
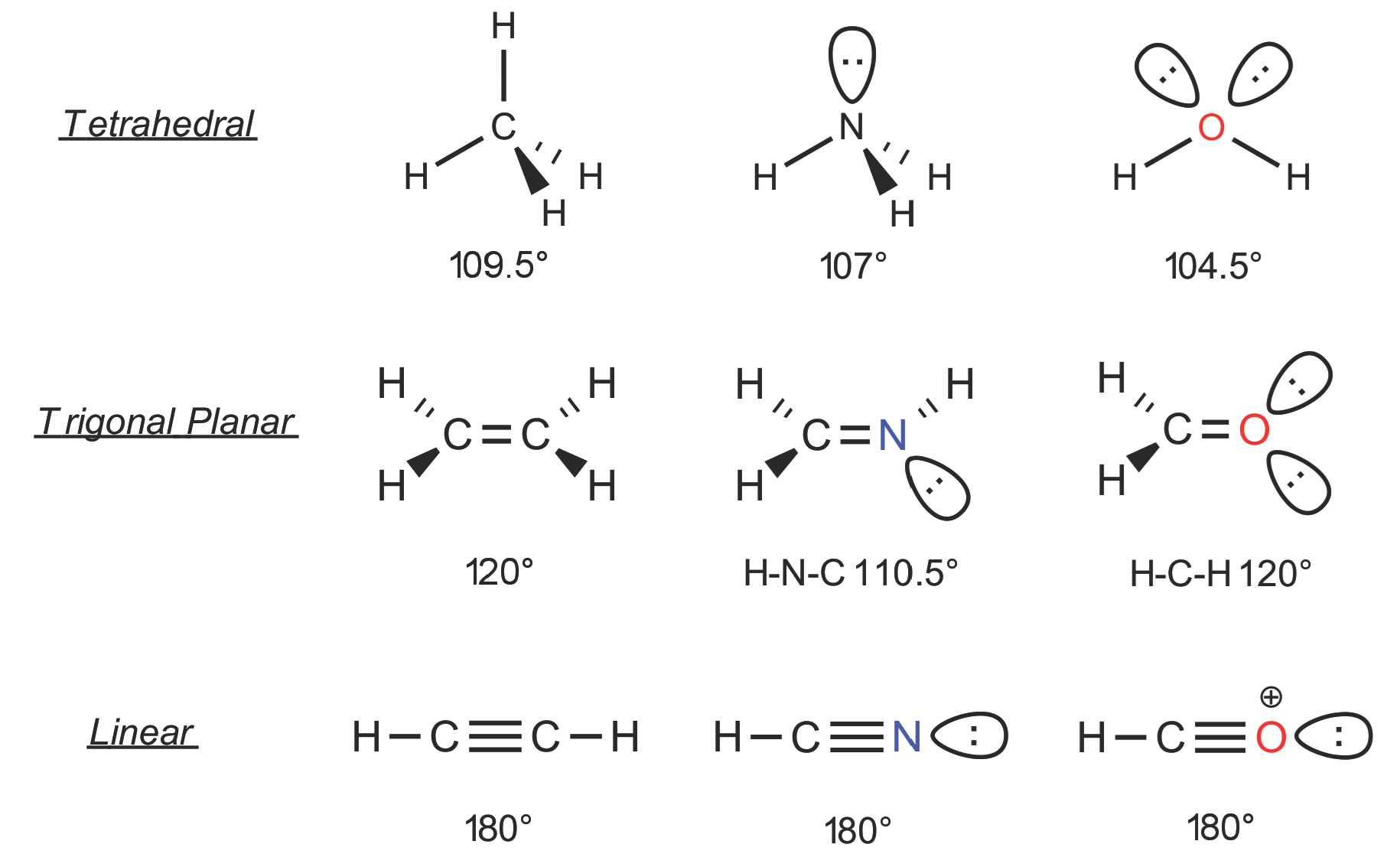
There are three important geometries displayed by carbon, and then nitrogen and oxygen with small deviations. When C is connected to other atoms by only single bonds it is said to be sp3 hydridized with a tetrahedral geometry. This is exemplied by the molecule ethane below in which all H-C-C-H bond angles will be close to 109 degrees. For nitrogen, which has a lone pair in place of one bond, the angle deviates away from 109 since the lone pair repels more; likewise for oxygen with two lone pairs. The shape around those atoms may be described as distorted tetrahedral (or trigonal pyramidal for N and bent for O).
When C (or N or O) are involved in one pi bond, the hybridization is now sp2 and the geometry changes to trigonal planar. Bond angles are approximately 120 degrees, since the three sigma bonds dictate the shape and the pi bond plays no role in that. This may be seen in the alkene ethylene below. Finally, when the central atom is involved in two pi bonds, the hybridization will be sp and the geometry will now be linear as in the alkyne acetylene, below.
Ethylene (sp2 C) trigonal planar
Acetylene (sp C) linear
Moving to Nitrogen and Oxygen we recognize the introduction of lone pair(s) which are known to take up more volume than bond pairs and so change these shapes slightly. For ammonia (N bonded to 3 hydrogens) and water (O bonded to 2 hydrogens) the tetrahedral shape still applies but with slightly different bond angles.
Similarly, for N and O in double bonds the shape is roughly trigonal planar but the associated lone pairs distort the angles away from 120. The molecules will, however, still be flat overall. For the linear molecules the N equivalent (nitriles) has the lone pair opposite to the alkyl group attached with the molecule still being linear overall.
Ethane (sp3 C) tetrahedral