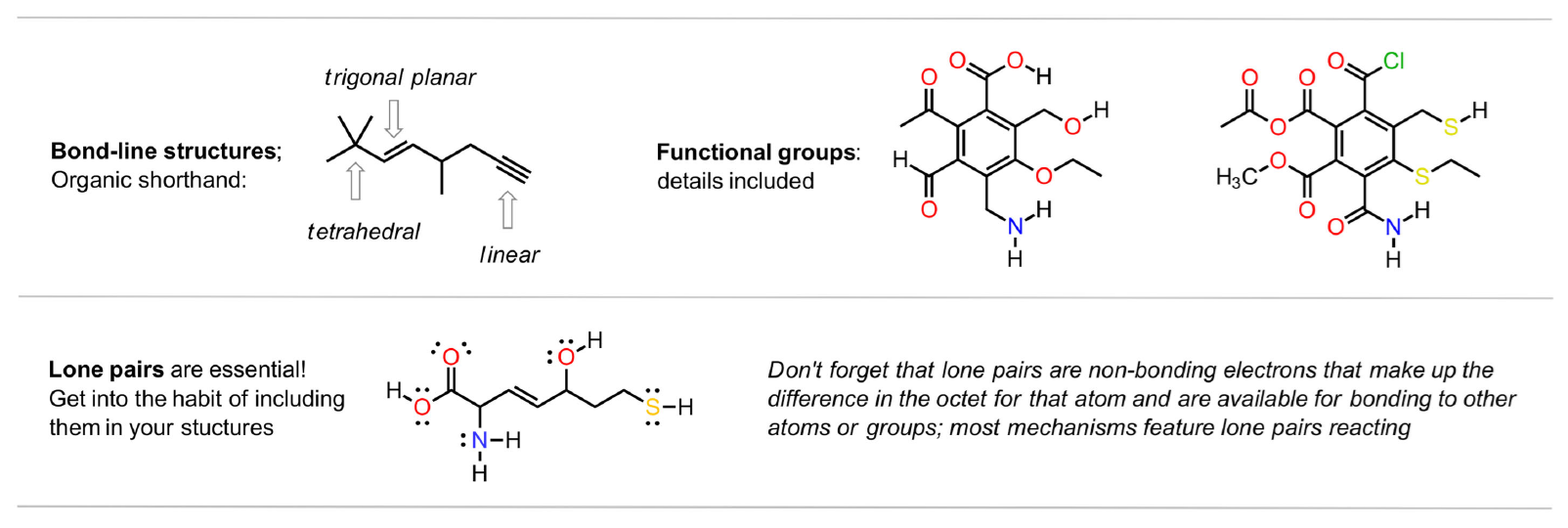
functional groups
Second semester groups found here
It is important to be able to identify functional groups in organic molecules since they are the entities that have function and undergo chemical change. There are around 15 important functions that must be memorized over the two semesters, many of them being used later in Biochemistry and Polymer Chemistry. Examples of first semester groups are below and both semesters are summarized in the video.
Alkyl Halides
Alkyl halides, in which sp3 carbon is bonded to fluorine, chlorine, bromine, or iodine are generally quite dangerous compounds to work with, however they find many uses in organic synthesis and polymer chemistry. Simple alkyl halides are used in substitution and elimination reactions, in which the polarized C-X bond makes them electrophilic, and the halogen serves as a leaving group. Alkyl halide polymers include polyvinyl chloride (PVC) used in house siding and plumbing pipes, as well as the perfluorinated teflon that is used as a non-stick coating on cooking equipment and water-sealed plumbing tape. An example of a simple alkyl halide is shown here.
1-Chlorobutane (1o alkyl halide)
Alkenes
Alkenes are organic molecules with a double bond betwen two adjacent carbons. A strong sigma bond between the two atoms is joined by a weaker pi bond above and below. They will be trigonal planar in shape around the functional group and serve as important nucleophiles in organic synthesis. They are typically made by elimination reactions in the first semester, and then by methods such as the Wittig reaction later on. The substitution pattern of the alkene plays a role in its stability and reactivity, with more attached alkyl groups stabilizing the alkene carbons through electron donation. An example of a simple alkene is given here.
Cyclohexene (disubstituted)
Alkynes
Alkynes are unsaturated organic molecules featuring a triple bond between two adjacent carbons made up of a strong sigma bond and two weaker pi bonds that occupy space away from the sigma bond axis. The sp hybrid carbons involved make attached protons on terminal alkynes slightly acidic, so strong bases may deprotonate to produce acetylide nucleophiles. The linear shape makes alkynes suitable for directing attached groups into opposite directions in space. Alkynes are produced by sequential elimination reactions from dihalides using strong bases like sodium amide. An example of a simple terminal alkyne is shown here.
1-Hexyne (terminal)
Alcohols
Alcohols are readily-available feedstock organic molecules containing the polar OH functional group. Depending on the nature of the alkyl group attached, these compounds may be soluble in water due the OH group’s ability to hydrogen bond. Alcohols are weakly acidic (pKa in the 16-18 region) so they may be deprotonated by strong bases to give alkoxide nuleophiles, which are then useful for making ethers. Primary alcohols are oxidized to aldehydes, and then carboxylic acids, where secondary alcohols give ketones. Tertiary alcohols are typically not oxidized under standard conditions. This can serve as a useful test for differentiating alcohol type
Cyclopentanol (secondary)
Ethers
Ethers are organic molecules in which a central oxygen is bonded to two individual carbon atoms. These molecules are inherently stable and serve as important solvents and find applications in medicinal chemistry and areas such as anesthesiology. Many examples occur in Nature, and the functional group is synthesized by such reliable reactions as the Williamson ether synthesis. The ether linkage is known to be robust and is generally stable in basic media. In acidic environments, protonation of the oxygen sets up a good leaving groups so substitution chemistry is possible. An example of a simple ether, the solvent diethyl ether, is given here.
Diethyl ether
thiols
Thiols are the sulfur equivalent of alcohols in which SH has replaced the OH group. The greater size and lower electronegativity of sulfur makes thiols more acidic (pKa around 10) than alcohols so they may be deprotonated by weaker bases. The lower electronegativity means S may be oxidized from 2 to 4 and then 6 in compounds such as sulfonic acids. This also makes thiol derivatives such as AcetylCoA important in Biochemistry as the S makes a better leaving group than its oxygen cousin. An example of a simple thiol, butanethiol, is shown here. This compound has an unpleasant odour and is added to the (odourless) natural gas supply to serve as an indicator.
Butanethiol
Sulfides
Sulfides, related to thiols, are the sulfur equivalent of ethers where the connecting sulfur has replaced the ether oxygen. The greater size and lower electronegativity of sulfur once again makes sulfides more chemically flexible than alcohols. The lower electronegativity of S means it may be oxidized from 2 to 4 and then 6 in compounds such as sulfoxides and sulfones, which themselves have broad use in organic synthesis. Sulfides occur in Nature, for example in the key biochemical cofactor S-adenosylmethionine (SAM), which is involved in biological alkylation reactions. An example of a cyclic sulfide, tetrahydrothiophene, is shown.
Tetrahydrothiophene